Galvanizing is one of the most popular methods of coating and protecting the metal. Galvanization is done by applying a protective zinc coating to steel or iron. This coating is mainly done to prevent rust and corrosion, which provides a longer useful life and increased safety.
Why Galvanize:
- Less Maintenance Required – Self-maintaining, thicker, and maintenance costs of these steel items are inevitably lower.
- Longer Life – On steel can last in excess of 50 years, continually protecting steel against corrosive elements.
- Lower Costs – Lower initial cost than a lot of other commonly specified corrosion protection coatings for steel.
- Quick Application – A full protective coating can be applied in minutes.
- Environmentally Friendly – The longevity of the maintenance-free coating provides environmental and economic benefits. Zinc exists naturally in the world so the zinc byproducts released into the atmosphere are not harmful.
Steel is the mainstay of modern construction and has been since the Industrial Revolution began, but left unprotected and exposed to the environment steel will begin corroding immediately. There are several ways to protect steel from the elements but none better than hot-dip galvanizing a process that bond’s molten zinc to the steel forming multiple protective alloy layers that protect the integrity of the steel for decades.
Click Here to Enlarge the Photo
Galvanizing protects our nation’s critical infrastructure and is one of the most cost-effective and sustainable methods available. Galvanized steel can be recycled indefinitely, has a low carbon footprint, and requires minimal maintenance. Hot-dip galvanizing is environmentally friendly since zinc is a natural and abundant mineral essential for life. In the industrial world, 30% of the world’s zinc supply comes from recycled sources, and 80% of zinc that can be recycled is reclaimed by dramatically prolonging the life cycle of steel and with lower to virtually no maintenance. The galvanizing process significantly reduces the energy and resources needed to manufacture, transport, and maintain these structures. Additionally, zinc is an abundant natural mineral that is 100% recyclable without losing any physical or chemical properties and when combined with steel there is a 70% recycled content rate.

Zinc has a special relationship with steel in the bonding process called hot-dip galvanizing. The zinc chemically bonds with the steel to form a series of zinc-iron alloy layers with the final layer being 100% zinc. These tightly adhered layers become extremely abrasion-resistant because the inner metallic layers are harder than the base steel, so the protective zinc coating actually becomes harder than the steel. Zinc also ages at a rate far slower than steel and it forms its own protective oxide carbonate film on its surface. The second additional shielding mechanism is zinc’s ability to galvanically protect the steel. When a scratch or abrasion occurs, the zinc coating will not be undercut by rusting steel because the steel cannot corrode adjacent to the zinc coating, even when exposed to the elements. This is why the use of zinc and the galvanizing process provides decades of maintenance-free protection for metal structures even in high usage areas and in harsh environments.
Corrosion and rust can result in large issues for safety equipment because of the potential weakening of the structure and the increase in health risks. If a worker was walking on a crossover platform that had rust forming on the base, there is a greater chance of the platform becoming unstable and dangerous. Additionally, rust and corrosion areas can become sharp and cause dangerous lacerations susceptible to infection. This is why top manufacturers coat their metal components using galvanization or powder coating.
Corrosion and rust can result in large issues for safety equipment because of the potential weakening of the structure and the increase in health risks.

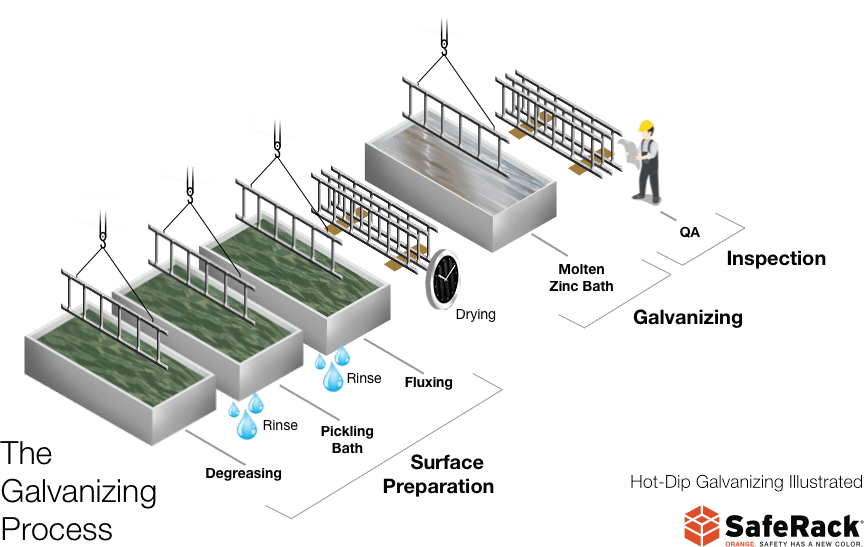
The most common galvanization method is hot-dip galvanizing, in which parts are submerged in a bath of molten zinc. There are three fundamental steps in the hot-dip galvanizing process
- Surface Preparation – The material moves through three cleaning steps to prepare the steel to be galvanized.
- Degreasing is the first step, removing dirt oil and organic residues. The structure is then rinsed.
- The second step is a mild acid or pickling bath, removing mill scale, iron oxide, and etch the steel. Then rinsed again.
- The third preparation step is fluxing, removing any remaining oxides, and then coating the steel with a protective layer to prevent any further oxide formation prior to galvanizing.
- Galvanizing – Once the structure is clean and dry it is dipped into a bath of molten zinc. As the material is immersed, the zinc will flow in and around the entire structure protecting all surfaces. While in the bath, chemical reactions take place between the zinc and the iron and the steel to form the series of zinc-iron intermetallic layers and an outer layer of pure zinc.
- Inspection – Inspection and Quality Assurance is the last step of the galvanizing process. A visual inspection of each structure ensures proper coverage and that the item meets the project requirements.
The less common method is electro galvanizing. The method does not use a heated bath of zinc, but an electrical current within an electrolyte solution. This transfers zinc ions onto the base metal. This is done by electrically reducing positively charged zinc ions to zinc metal which are then deposited on the positively charged material. A big advantage of this process is a uniform coating. However, this coating tends to be thinner than the dipping method, which could lead to a less rugged shell.
Other Industrial Coating Methods
There are other well-known methods of coating metal for industrial use aside from galvanizing. These methods include powder coating, painting, and staining. Each has its own benefits depending on the application. Many manufacturers have a two-step process of powder coating over galvanized metal. This gives a highly durable finish that is also aesthetically pleasing.
Powder coating, like galvanizing, is one of the top methods used in industrial metal components. Powder coating is the method in which a powdered substance is put through an electrostatic charge, typically from a spray gun, and the powdered particles are changed so that they attract to the base metal component and adhere a strong bond. Powder coating provides a durable and tough finish that is resilient and reduces the damage caused by impacts, severe weather, and chemicals. It also has a low probability of chipping and scratches.
As you may already know, painting and staining is done using a liquid and is not as durable as galvanizing or powder coating. The benefits of painting or staining are the ability to get a clean finish in many colors and looks. Chips, scratches, and discoloration can happen over time, especially in harsher environments such as foundries or ports. They tend to not have a high threshold for heat, and saltwater and chemicals can easily break the coating down or cause discoloration.
The difference in Powder Coating vs Paint – Pro, Cons, and Recommendations.
The difference in Powder Coating vs Hot Dip Galvanizing – Pro, Cons, and Recommendations.
Did you know: Do you need a tetanus shot if cut by a rusty object?
A common misconception about getting cut by rusty objects is that it’s the rust that causes the infection, but it’s a type of bacteria, Clostridium tetani, that’s on the rusty object that causes the infection. This bacterium is ubiquitous in the natural environment — spores lurk in soil, dust, and in animal intestines and feces. The rusty metal scenario isn’t the only way this disease spreads. Any deep puncture can become infected with Clostridium tetani, as can burns, torn flesh, punctures from needles during drug use, animal bites and scratches, or other wounds contaminated with human and animal feces or saliva. 1
SOURCES:
- http://goaskalice.columbia.edu/answered-questions/cut-rusty-metal-do-i-need-tetanus-shot
- https://www.nace.org/Corrosion-Central/Corrosion-101/Galvanic-Corrosion/